The purpose of this post is to examine whether electromagnetic fields could follow a chiral geometry in dendrites. There are three physical things that need to be chiral for chiral geometry to occur in dendrites.
1. Electric fields
2. Water
3. Spines
This post looks at each thing in turn then does some simple maths to determine whether it’s possible for an electromagnetic field to have chiral geometry in a dendrite. The post concludes that varying rates of oscillations can be used, to vary the period of a chiral field.
Chiral Waves
An electromagnetic wave can be chiral when it carries a nonzero angular momentum (Zhang 2011). Subwavelength surface plasmon polaritons (SPP) waveguides, are analogs of optical fiber waveguides, with waves carried on the surface of a metal wire. These wires must have nanometer-scale cross sections and micron-scale propagation lengths. There can be strong coupling between proximal quantum emitters and nanowire SPPs. SPPs also require unidirectional subwavelength light sources, which excite SPP modes with light incident to the nanowire. By controling the phase or polarization the incident excitation source can generate coherent superpositions of nanowire SPPs.
Zhang (2011) found TM0, HE1 waveguide modes are excited for parallel polorisation and TM0 and HE-1 waveguide modes are excited for perpendicular polarization.

DIagram (Zhang 2011) Showing how incident light creates different modes. (B) TM0, (C) HE-1 modes are excited for parallel incident polarization, and (D) HE-1, (e) HE2 modes. both TM0 and HE-1 modes are excited for perpendicular polarization. However perpendicular excitation couples at less than 10% of the rate.
Zhang (2011) also found that chiral SPPs could also be generate three fundamental waveguide modes of -HE1, HE1 and (the fundamental) TM0 mode illuminating a nanowire at one end with linearly polarized light at 45 degrees with respect to the nanowire axis. The maximum coupling was found at 40 degrees. The chirality disappeared when the incident polarization was parallel or perpendicular to the nanowire. Angular excitation gave rise to chiral surface plasmons which were observed experimentally using fluorescence imaging of the nanowire evanescent field, meaning the SPP produced a field external to the wire.

Diagram (Zhang 2011) showing the relation between radius and period. Wavelength of 30um. (A) Surface charge density plot on a 60nm Ag nanowire with 5um length (B) shows the surface power (C) shows the wavelength (period) of the chiral field for different diameters.
The surface charge density on the wire is distributed in a chirally, with a period of 1.8um on a radius of a 60nm wire with 632.8 nm light. The chiral period increased with a larger diameter
The graph above shows the is a positive relationship between the radius and the chiral period. The equation to calculate the radius from the wavelength is based on the wavelength as follows:-
R ∼ λ √ε
The radius that can be support a chiral SPP is based on the wavelength and permittivity ε. As a rule of thumb with a permittivity of 1 then the radius is the same as the wavelength.
Chiral Water
Dendrites are filled with water therefore an important consideration is whether water will form chiral structures.
Ho (20112) photographed stable chiral water clusters, in distilled water at room temperature, tens of nanometres in diameter and tens of micrometers in length, using a transmission electron microscope (TEM) and an atomic force microscope (AFM). According to Ho (2012) these structures remained stable for hours or weeks.
The water appeared to form from a common structure of small spheres tens of nanometres in diameter, lined up in strings, that are further aggregated into rods. Two of which wound around each other into a double-helix.

Photo of double helix formed by stable water clusters (Ho 2012)

A diagram (Ho 2012) showing a chiral water structure, and location of small spheres.
In this study the concentration of NaCl was ~10-7M. At higher concentrations the ion dipole interactions dominated. The point at which dipole-dipole interactions dominate is found experimentally to be ~10-4M. Below a transition point the water molecules attract each other to form clusters.
These water clusters have been theoretically shown to have interesting “coherent” properties (Del Giudice 2006, Czerlinski 2015), which have a close relationship with quasi static properties identified by Le Blonde (1973).
Another point to make here is that whilst Ho (2012) found long lived stable water structures, previous posts indicate that long lived stable or unstable water is unwanted in neurons. What would be preferred is controllable short lived stability. It was shown by (Omelyan 2016) that oscillating charges would temporarily change the orientation of water dipoles and therefor could temporarily chnage the water cluster.
Chiral Cerebellum Spines
The third requirement for electromagnetic chiral structures in dendrites is that there is some kind of electrical source that can cause chiral water and electromagnetic fields to be created. A prime candidate for investigation would be spines on cerebellum Purkinje dendrites.
O’Brien (2005) has shown the spines on cerebellum Purkinje dendrites are arranged in a chiral geometry. This geometry is preserved from fish to mice. Purkinje cells, dendrites and spines in the cerebellum have relatively simple architecture and circuitry.
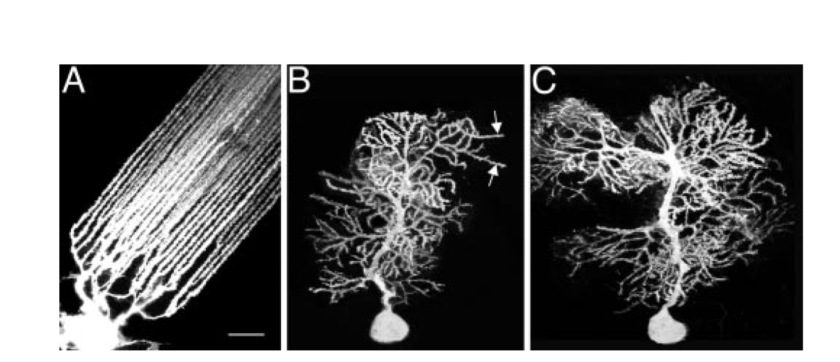
Diagram (O’Brien 2005) showing the dendrites of the Mormyrid fish (A), weaver mouse (B), and wild-type mouse (C).
O’Brien (2005) looked at mormyrid fish and mice. The fish had a lower density of spines along the distal dendrites and a more regular cerebellum than mice. The fish dendrites, were straighter, thinner and more uniform than the mice.
The fish dendrites traced a single and double helical path in the distal dendrites.

Photo (O’Brien 2005) showing (A) two adjacent shafts from the same fish Purkinje cell, and (B) the trace showing the double helix and single helix of spines around the shafts.
Linear arrays of spines were most obvious in the regions of low spine density, where they oriented at angles to the axis of the shaft (typically 10º). The periodicity of a single helix was typically 1.35um, 1.4um, 1.6um, 1.7um. The periodicity of a double helix was typically, double the size, of 2.7um and 2.8um.
The chiral spines were also found mouse, but were more randomly oriented.

Photo (O’Brien 2005) showing the helical spines around the dendrites of the weaver mouse.

Photo (O’Brien 2005) showing the helical and double helical spines around the dendrites of a wild type mouse.
O’Brien’s (2005) proposal is that the spines of Purkinje cells are formed by an ‘‘intrinsic mechanism,’’ “independent of interactions with their presynaptic partners”. This organisation means that the helical pitch between the spines ensures that each spine is located at a different level along the shaft. This will evenly space out spines and mean that each spine contacts to a synapse on a different axon. This separation would create a more uniform distribution of electrical charge.
Another study (Yadav 2012) found evidence of spine clustering in apical dendrites of neocortical pyramidal cells. A statistical analysis of human neonatal cortical pyramidal neurons concluded (Morales 2012, 2014) that dendritic spines were positioned randomly nor followed chiral patterns. However, this study did not look at distal branches of apical or oblique dendrites, or mature adults.
The Design of Chiral Fields in Cerbellum Dendrites
This final section looks at whether chiral electric fields could exist in distal dendrites.
There are a few similarities worth noticing between the three examples above, The scale of all the three examples above are very similar. There is a correlation in geometry between the water spheres and spines. Both the electric fields and the spines are angled into the wire or dendrite.
This next section looks whether the period of the helix in the dendrite can accommodate a chiral electromagnetic field.
The electric example above gives the following equation that relates the radius to the wavelength. This shows the permittivity effects and the radius and previous posts have shown that permittivity can be varied in water.
R ∼ λ √ε
The following calculations will use the wavelength of the calcium ion (Ca+2) because that ion is found in distal dendrites. Ca+2 has a wavelength of 286nm. The calculation will also look at at three different realistic values for permittivity, based on the permittivity in bulk water, measured in neurons, and modelled in an oscillating TIP4 water model (Omelyan 2012).
With a permittivity of 80 (bulk water) the field radius would be 286nm x 9 = 2574nm (diameter 5um) , which would be five times greater than the 500nm radius of a dendrite. This would have the effect of creating a wave outside the neuron.
With a permittivity of 1.38 (measured in neurons – Rappaz 2005) the radius would be 286 X1.17 = 335 (diameter 670nm), which would fit just inside the diameter of a dendrite. Based on the graph, the period of the wave would be > 30um.
In order to get shorter periods the permittivity would need to be reduced to below 1. With a permittivity of 0.01 this would create a radius of 28nm (1.10 of the radius) and reading off the graph a period of 1-3 um. This period size would align with the chiral spines on a cerebellum. This low level of permitivity could be created by rapidly oscillating ions.
These approximate figures show that the step reduction in permittivity in water due to oscillation could change the field in a dendrite from a longitudinal-like-field to a shorter chiral field that could couple with the chiral spines in the cerebellum dendrite.
Importantly, the rate of oscillation could be varied to change the permittivity, between 1.38 and 0.01, which would then keep the wave inside the neuron and change the period of the electromagentic field from 30um to 1um. Thus the rate of oscillation could be used to change the path between different spines in the distal cerebellum dendrite.